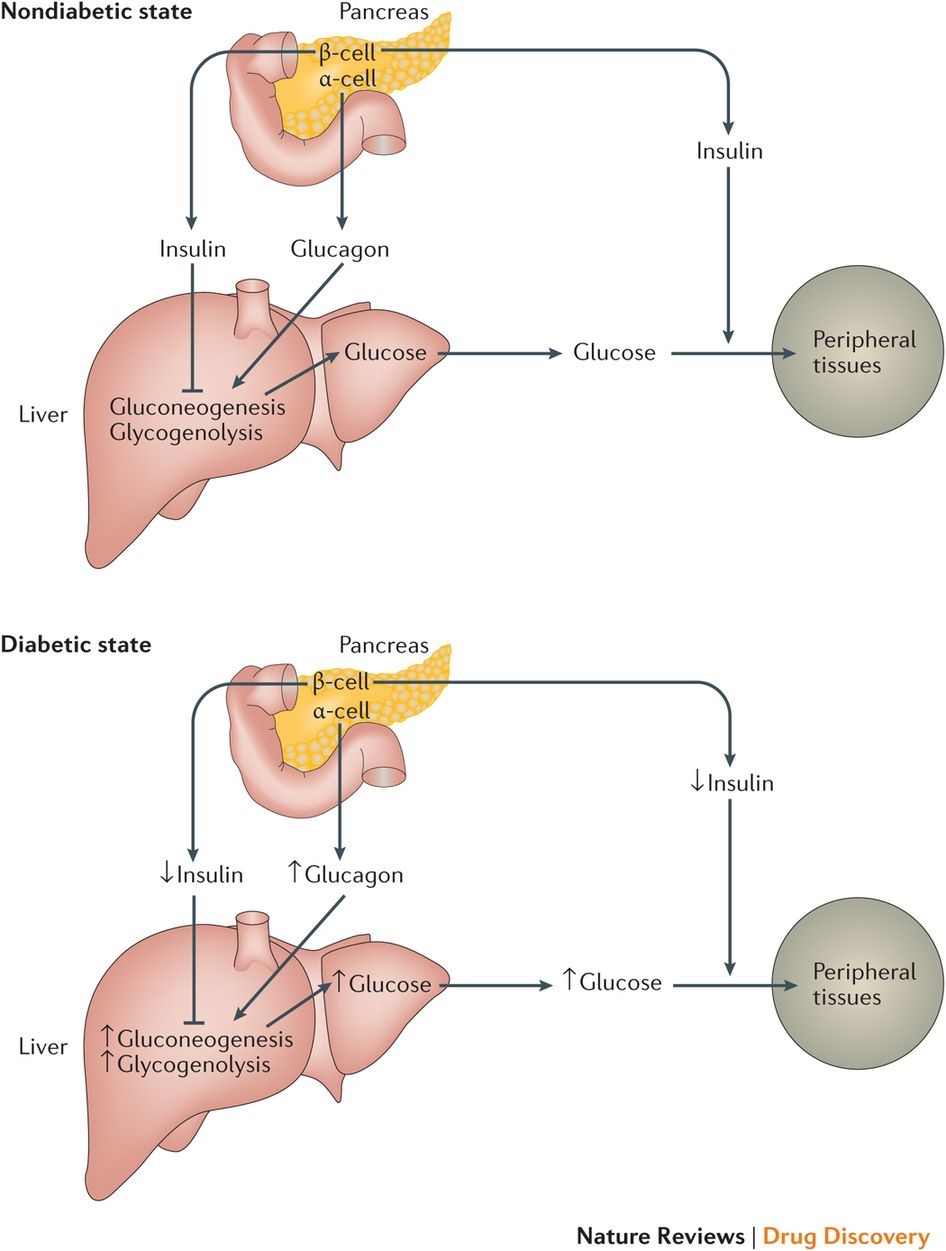
Type II diabetes is a chronic condition in which the body cannot maintain a normal blood glucose level. This occurs when the body resists the effects of insulin and eventually doesn't produce enough insulin to maintain healthy blood sugar levels. Insulin, which is produced by the beta cells of the pancreas, is an important hormone that regulates the movement of glucose into the body's cells as well as inhibits the release of glucose from the liver into the blood when blood sugar levels are high. The resistance and decrease of insulin in diabetes patients cause high blood sugar levels since glucose stays in the blood instead of being taken up by the cells, also called the effectors.
Glucose Metabolism:
Glucose, which is stored in the body as glycogen, is kept in the liver and is broken down to glucose and then released into the bloodstream to raise glucose levels. This process is regulated by the glucagon released from pancreas alpha cells when blood glucose levels are low. The glucose released to the bloodstream is from either glycogenolysis, break down of the glycogen, or gluconeogenesis, a process that converts non-carbohydrate molecules such as amino acids, fat or lactate, and pyruvate into glucose. The liver is the primary location of gluconeogenesis and glycogenolysis.
Insulin and glucagon work synergistically in glucose metabolism. Blood glucose levels increase after a meal which then stimulates the release of insulin. This release promotes the uptake of glucose into the effectors to lower blood sugar levels.
The major effectors that take up glucose from the blood are the liver, skeletal muscles, and adipose tissues. When insulin levels are high, effectors increase the uptake of glucose which can lead to one or more of the following: increased glucose uptake by membrane transporters, increased breakdown of glucose to provide energy or cause glucose to be converted to glycogen to be stored in the liver.
Insulin works through cell surface receptors called tyrosine kinase-linked receptors. When the receptor becomes activated by the binding of insulin, the receptor then phosphorylates a number of intracellular proteins which generate a biological response. In resting skeletal muscle and adipose tissue, insulin mobilizes the GLUT4 transporter for diffusion of glucose. GLUT4 can only be triggered by insulin. In working skeletal muscles, typically during exercise, insulin is not required for the uptake of glucose because physical activity mobilizes the transporter GLUT4. This is why physical activity is an important factor to help maintain blood sugar levels.
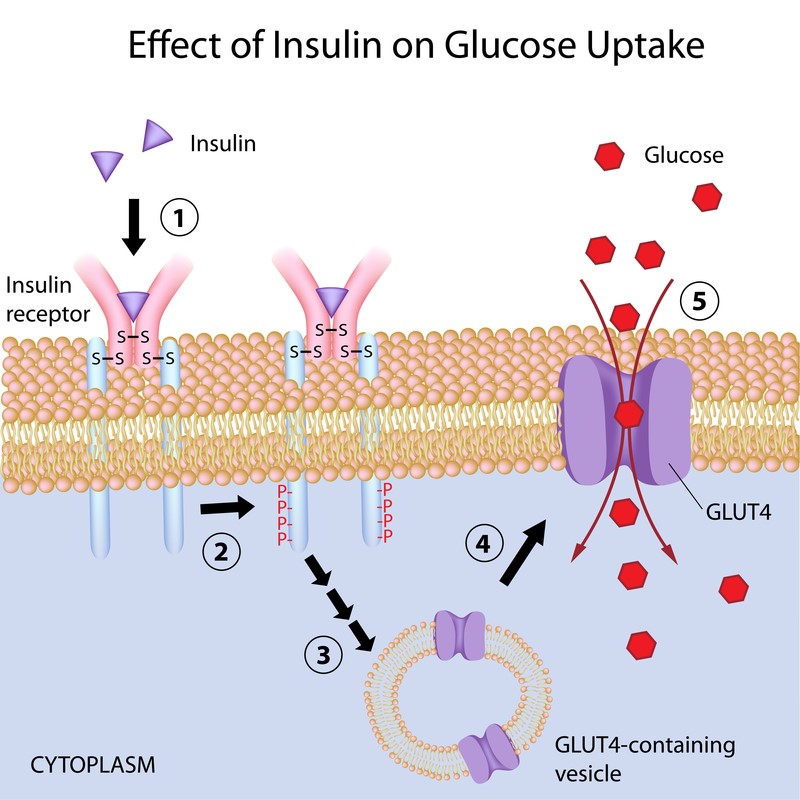
The liver, unlike adipose tissues and resting skeletal muscles, can uptake glucose without insulin. The liver uses a different transporter (GLUT1, 2, or 3) that resides permanently in its plasma membrane. Due to the fact that the liver has Glucose-6-phosphatase (G6Pase), it is the only organ that can release a significant amount of glucose into the blood. The binding of insulin to the receptor on the hepatic cell triggers the inhibition of glucose release into the blood and downregulates the liver's gluconeogenesis and glycogenolysis process. The inability of insulin to suppress hepatic glucose production is a key defect found in type II diabetes.
The normal age of onset for type II diabetes is over 40. Normal blood sugar levels range from 72 to 99 mg/dL. In patients with prediabetes, the range is from 100 to 125 mg/dL and for those with full blown diabetes, higher than 126mg/dL. When blood sugar levels are consistently high such as in prediabetes and diabetes, the long-standing glucose in the blood can damage the vessels that supply blood to vital organs. Other dangers of hyperglycemia include increased risk of heart disease, stroke, kidney disease such as CKD, vision problems, and nerve problems. Hypoglycemia (below 70 mg/dL) can also occur in patients with diabetes. This can occur with medications that increase insulin levels, taking too much medication, and skipping meals. Although mildly low blood sugar levels are somewhat common for people with diabetes, severely low blood sugar levels can lead to seizures and nervous system damage.
Causes
1) Inflammation
Although several genetic and lifestyle factors can contribute to insulin resistance, research has pointed out that
inflammation plays a key role. Insulin resistance and diabetes are ultimately caused by chronic inflammation in the
stomach, pancreas, liver, muscle, and fat tissues. Inflammation markers including C-reactive protein (CRP),
interleukin-6 (IL-6), and tissue necrosis factor (TNF) can modulate insulin signaling pathways causing the tissues to
become less sensitive to insulin. In muscle and adipose tissue, IL-6 decreases the number of GLUT4 glucose
transporters in the cell membrane and reduces the expression of insulin receptor substrate-1 (IRS-1), the key
components of insulin signal transduction pathway. In skeletal muscle, there is an inversely linear relationship
between maximal glucose disposal rate and muscle TNF-α levels. 3 Inflammation markers in the hepatic cell activate
gluconeogenic enzymes causing enhanced gluconeogenesis in the liver.
The chronic inflammation that contributes to insulin resistance affects not just insulin target tissues; it is at a systemic
level affecting the whole body with increased levels of inflammatory markers circulating in the bloodstream. Research
has identified that such systemic inflammation originates from the digestive tract. Gut dysfunction and failure of gut
homeostasis causes the pathogenesis and progression of systemic inflammation due to the exacerbation of local
and systemic immune responses. The inflammation markers generated from the gut affects the liver through the
portal vein circulation and further affects the muscle, adipose and other tissues through systemic circulation.
2) Candida Fungal and Other Types of Infections
Fungal infections in the liver can also cause insulin resistance leading to type II diabetes. Secreted aspartyl
proteinases (SAP proteins) produced by Candida can destroy the insulin receptors on the cell surface of the liver
and block insulin signal transduction. To compensate for the reduction of functional insulin receptors in the hepatic
cell surface, the pancreas must secrete a higher concentration of insulin to maintain blood sugar levels and insulin
resistance occurs. The fungus that infects the liver usually comes from the digestive tract. The fungus in the digestive
tract can also spread to the pancreas causing pancreas irritation and inflammation. Chronic hyperproduction of
insulin and pancreas inflammation can cause early degeneration of the beta cells. Eventually, the beta cells can no
longer produce enough insulin to overcome insulin resistance. As a result, blood sugar levels rise to an above normal
range and patients become fully diabetic.
Pancreatic irritation by mycotoxins can cause pancreatic inflammation and injury. Insulin over production in
combination with inflammation, and injury can lead to the destruction of the pancreas. Eventually, the pancreas can’t
produce enough insulin to overcome insulin resistance and type II diabetes may occur. In addition to elevated blood
sugar levels, the mycotoxins can also cause symptoms of increased thirst, extreme hunger, frequent urination,
fatigue, and weight loss. Some patients also experience constipation because mycotoxins can cause GI blood vessel
inflammation and affect blood circulation. This leads to decreased GI smooth muscle contractions.
Candida fungal infections of the pancreas can weaken the immune system and increase susceptibility to other
infections, including gram-positive bacteria, gram-negative bacteria and mycobacteria. These coinfections can also
cause pancreatic inflammation and injury accelerating the destruction of the pancreas causing type II diabetes.
These coinfections can also cause constipation and other GI symptoms.
Genetics can play a role in the development of diabetes due to specific DNA that affects how the body makes insulin. Researchers are currently still trying to pinpoint the specific gene that carries this risk.
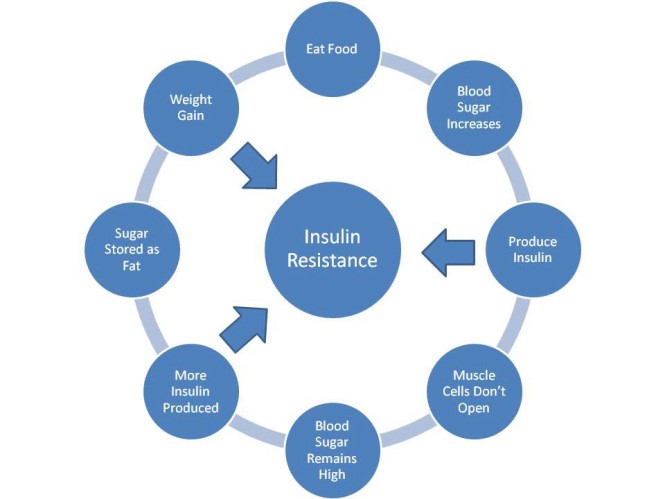
Obesity is a big risk factor in the development of type II diabetes since poor eating habits go hand in hand with elevated blood sugar levels. When a patient ingests a lot of high sugar foods, the result is high glucose levels in the blood. The pancreas then needs to exponentially increase its secretion of insulin to maintain a normal blood sugar level. Over time, such high blood insulin levels can cause the insulin receptors to lose their affinity to insulin (insulin resistance). The cell signal transduction then no longer communicates the movement of glucose into the cells and no longer regulates glucose release from the liver. To compensate for the reduction in the response of insulin receptors to insulin, the pancreas has to produce higher levels of insulin, which is a symptom of insulin resistance. This puts higher demand on the insulin-producing beta cells in the pancreas, often setting the stage for diabetes. This stage of the condition is usually referred to as prediabetes in clinic. Chronic overproduction of insulin can cause the beta cells to burn out and stop producing insulin. As a result, blood sugar levels rise to an above normal range and patients become full-blown diabetic.
Hyperlipidemia is also common among types II diabetes patients. In addition to the sugar metabolism disorder, the patient may also have high cholesterol, triglyceride, and VLDL levels. Recent research has shown that elevated blood sugar levels may actually be caused by hyperlipidemia. The abnormal blood lipid levels may inhibit the interactions between insulin receptor proteins and affect signal transduction. Such interference can cause insulin to work less efficiently with the insulin receptors on the hepatic cell surface (insulin resistance). In order to compensate for the reduced efficiency of insulin, the pancreas has to secrete higher levels of insulin to maintain normal blood sugar levels. Patients become insulin resistant and are then classified as pre-diabetic. The liver plays a key role in the lipid metabolism. Many type II diabetes patients who have hyperlipidemia also have elevated liver enzyme levels (AST/SGOT and ATL/SGPT). In these patients, their poor liver function causes hyperglycemia which triggers the insulin resistance. When the beta cells eventually burn out and stop producing insulin, blood sugar levels rise to an above normal range leading to the development of type II diabetes.
Symptoms
The symptoms of diabetes typically develop slowly as the disease progresses. Increased thirst is a common symptom as excess glucose builds up in the bloodstream causing fluid from surrounding tissues to be pulled out. This effect leaves patients thirstier and as a result, polyuria is another common symptom. Increased hunger is also a symptom since there isn't enough insulin to move sugar into the body's cells, therefore, neglecting the muscles and organs of energy. This effect can trigger hunger since the body feels it has no energy. This effect can also cause fatigue in diabetes patients since the cells are deprived of sugar, which results in decreased energy. Blurred vision can also occur with hyperglycemia when fluid is pulled from the lenses of the eyes. If the blood sugar levels can't be maintained, patients can develop complications including cardiovascular disease, kidney failure, foot neuropathy and retinopathy due to damage to the eye.